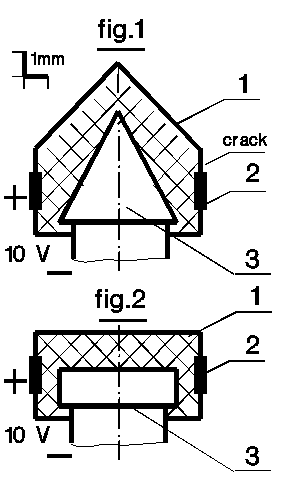
 Electrolysis of conical (fig.1) and cylindrical (fig.2) samples will be
compared here. Reasons for the special attention to the conical cathode (3)
sample had been presented at the bottom of
numerical simulation page.
Electrolysis of conical (fig.1) and cylindrical (fig.2) samples will be
compared here. Reasons for the special attention to the conical cathode (3)
sample had been presented at the bottom of
numerical simulation page.
 Electrolysis of conical (fig.1) and cylindrical (fig.2) samples will be
compared here. Reasons for the special attention to the conical cathode (3)
sample had been presented at the bottom of
numerical simulation page.
Electrolysis of conical (fig.1) and cylindrical (fig.2) samples will be
compared here. Reasons for the special attention to the conical cathode (3)
sample had been presented at the bottom of
numerical simulation page.
In contrary to the sample of main page, as ion-conducting substance (electrolyte - 1) the sodium stearate (C17H35COONa - the main part of soaps) was employed. Its melting point is 250oC and at about 180oC the substance provides 0.05-0.1 mA Na+current through the native samples. Anion current is impossible (long hydrocarbon tail). Na cannot leak to atmosphere and diffuse through electrodes (as hydrogen could do). The substance is strength enough and brittle that usually prevent plastic deformations of the samples. Electron conductivity of the substance is zero.
Surfaces of the steel cathodes were ground to give an white metal finish, and cleaned by spirit. Accuracy of the conical tip was about 0.2mm. Equal areas of the both cathodes were covered by electrolyte in course of its crystallization from liquid phase. Heat of the crystallization was transferred into the electrodes that defined the outer surface shape (small heat-conductivity of electrolyte limited electrolyte layer thickness above the steel surfaces).
Initially, full charge (before saturation) passing through conical sample was about 2 times of cylindrical one (about 0.05 C). Then the cathodes were cleaned without any abrasives, and covered by electrolyte again. Cylindrical sample results didn't change upon it, but both conductance and full charge through conical sample increased (was the steel surface refined by ion cumulation movement along cathode's slopes?). One more such cycle had led to 5 times increasing of initial conductance and more than 10 times of initial charge. It was accompanied by big plastic deformation of electrolyte. Probably this deformation mixes electrolyte layers near the cathode surface that results in the charge increasing, but it don't explain the current increasing at the standard temperature.
On the other hand, when the temperature was decreased to limit initial current to 0.05-0.1mA, the current would stop soon (charge was about cylindrical sample one). However, the stop takes place only after a crack appearance. The crack directing along sample axis is pointed out at fig.1.
A conical sample electrolyte crack appeared also after first and second electrolysis, but it took place during several days storing at room temperature. The crack position was usually near cathode cone foundation.
These facts are explained if the stress developing inside conical sample is much bigger than in cylindrical case.
Next stage should employ a thin (less 1mm to prevent the moderate thickness electrolyte destruction) wire-cathode with very well-shaped tip. I've got an idea how to do it but would prefer a collaboration if somewhere the wire is. More strength and brittle electrolyte such as beta-alumina should be employed also.
( more systematic description of the Part I results in .doc paper format or in .pdf format)
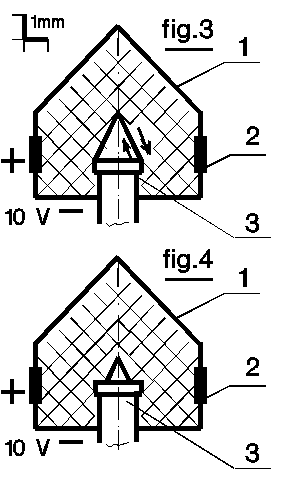 Check your intuition. Whether the electrolyte cap (1, fig.1, 3, 4) is put on
the cathode (3) during the electrolysis or it is taken off. The cone tip
(fig.3) sharpened by a fine sandpaper is blunt after the electrolysis.
It does not take place if a shelf is placed around the cone foundation
(fig.4). The last case leads to the biggest electrolyte destruction near
the shelf. So transversal forces balance for cathode and inner electrolyte
surface pointed out in fig.3 is quite correct.
Check your intuition. Whether the electrolyte cap (1, fig.1, 3, 4) is put on
the cathode (3) during the electrolysis or it is taken off. The cone tip
(fig.3) sharpened by a fine sandpaper is blunt after the electrolysis.
It does not take place if a shelf is placed around the cone foundation
(fig.4). The last case leads to the biggest electrolyte destruction near
the shelf. So transversal forces balance for cathode and inner electrolyte
surface pointed out in fig.3 is quite correct.
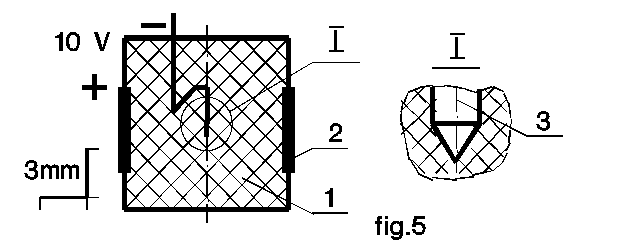
The fig.3-5 show that the experiments of part I were repeated with smaller cathodes down to 0.5 mm in diameter. The electrolyte dimensions are not changed, and strength of the samples should be much bigger. Nevertheless, the above results are repeated again except the crack character. Several small cracks (or bubbles) rather than the big one come to electrolyte surface.
For wire-cathode sample (fig.5) the crystallization quality cannot be guaranteed so far. There is some porosity of native samples here. Nevertheless, these results mean absence of ion cumulation limits, and provide opportunity of ultrahigh pressure generation. The pressure should be much bigger than the one developed by standard mechanical high pressure technology (Bridgman anvils, for example). The methods lead to 25-30% material volume decrease to date. Even 2 times static reduction of interatomic distance should provide pycnonuclear reaction onset (see main page nuclear appendix).